Lewis acid/base cooperativity
Lewis acid/base cooperativity
Applications of two-centre Main Group systems for small molecule activation and catalysis
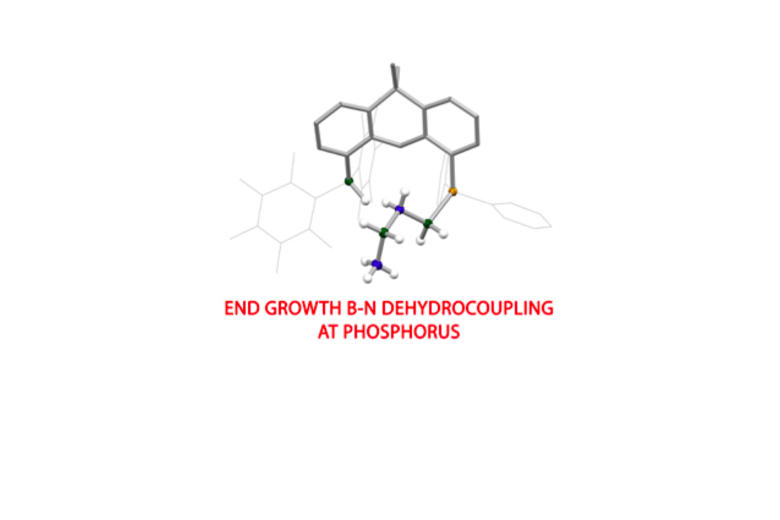
In addition to single-site approaches for small molecule activation, we are also examining systems in which the HOMO and LUMO are localized on different atoms. Geometrically constrained Lewis acidic and Lewis basic sites offer a cooperative two-site approach to the activation of kinetically challenging substrates. Within this area, our research covers two broad strategies: (i) the use of molecular Main Group systems containing highly polar bonds for two-site (typically 1,2) activation of kinetically inert substrates; and (ii) applications of pre-organized (phosphine/borane) frustrated Lewis pairs in small molecule activation and catalysis.
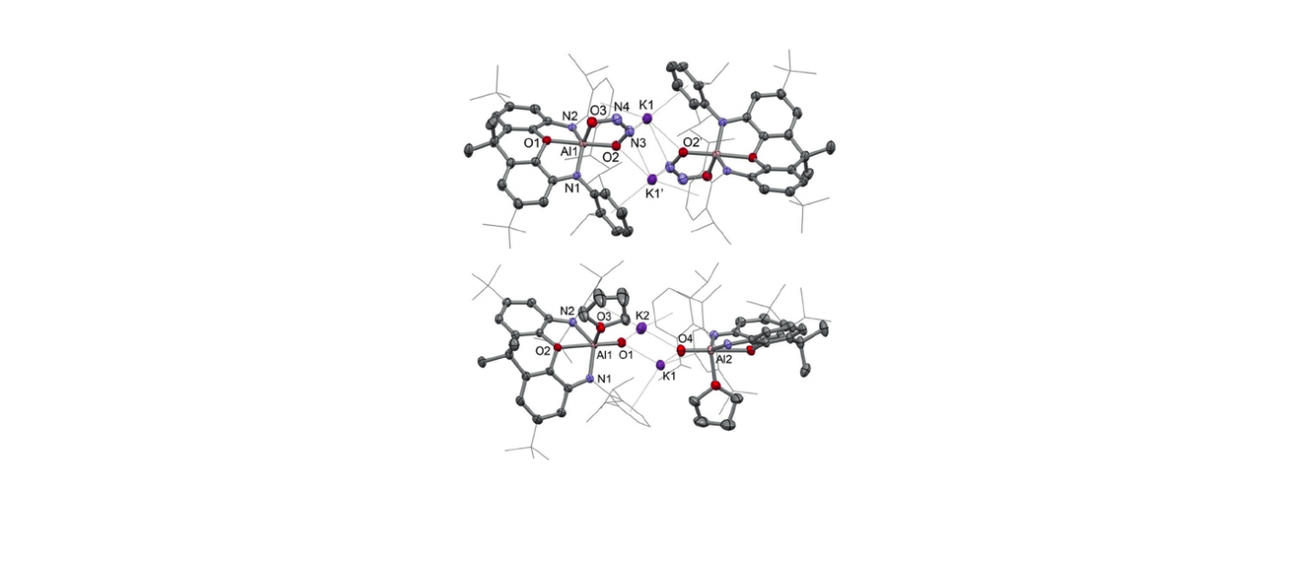
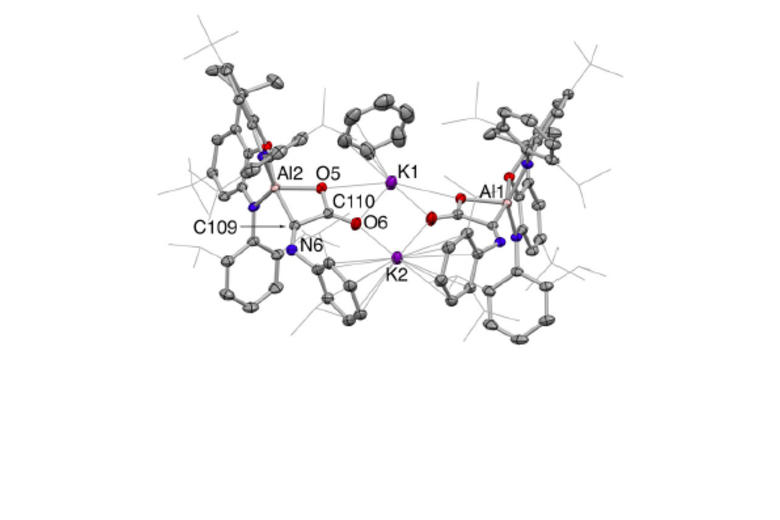
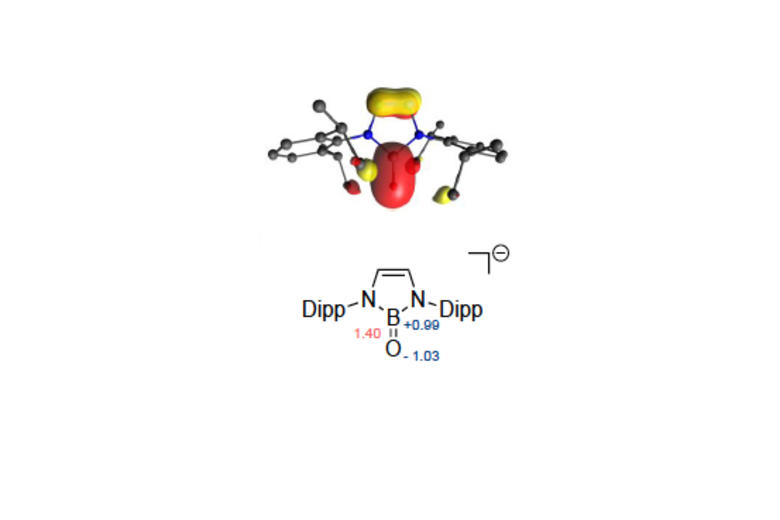
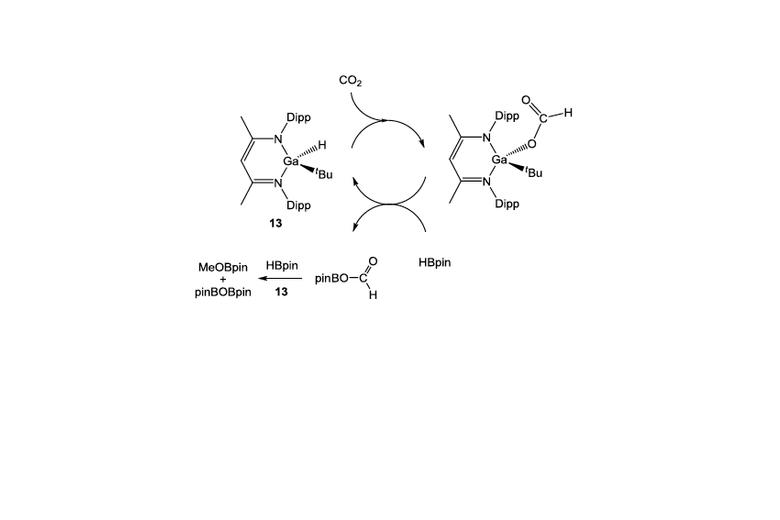
Recent highlights in these areas include: (i) molecular aluminium oxide and imide compounds (containing highly polar Al-O or Al-N bonds), which are not quenched by aggregation and which activate substrates including hydrocarbons, dihydrogen and CO; (ii) synthesis of an anionic oxoborane containing a B=O double bond and its action as an oxide ion transfer agent in 'transition-metal like' fashion; (iii) activation of protic, hydridic and apolar E-H bonds in 1,4-fashion by a gallane complex and its implication in the catalytic reduction of carbon dioxide; (iv) frustrated Lewis pairs based on a preorganized xanthene backbone which are capable of the reversible activation of dihydrogen in solution at room temperature and which can be employed in a range of catalytic dehydrogenation processes.
Selected publications: