Lewis acids for binding/detection
Lewis acids for binding/detection
Design and synthesis of Lewis acids and acid/base pairs for analyte binding, detection and delivery
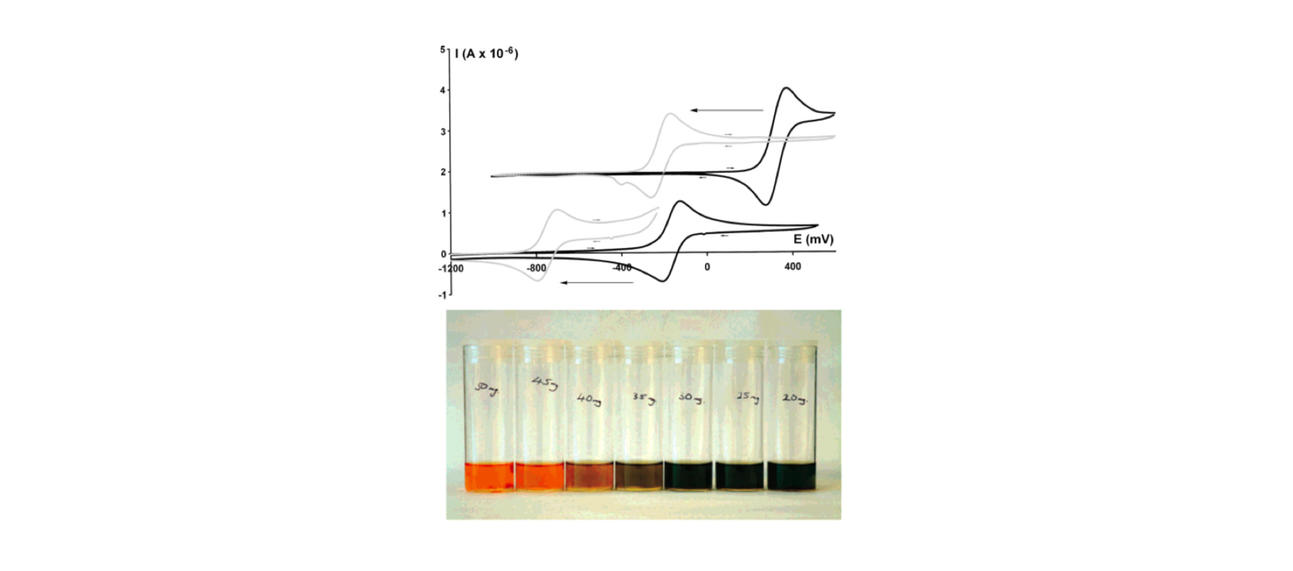
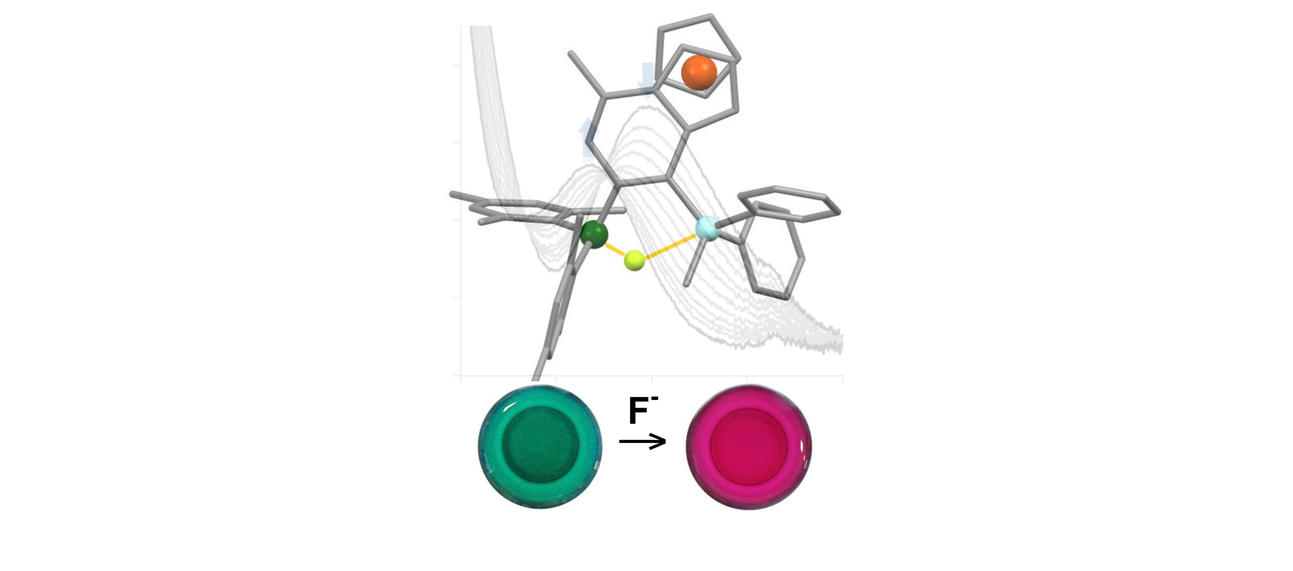
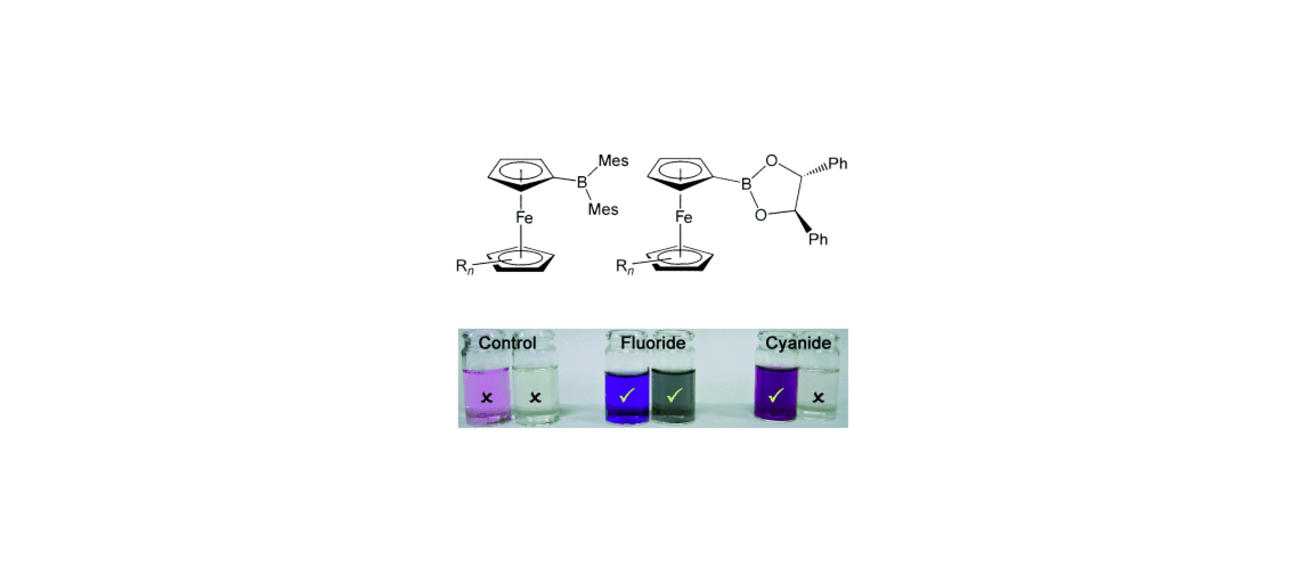
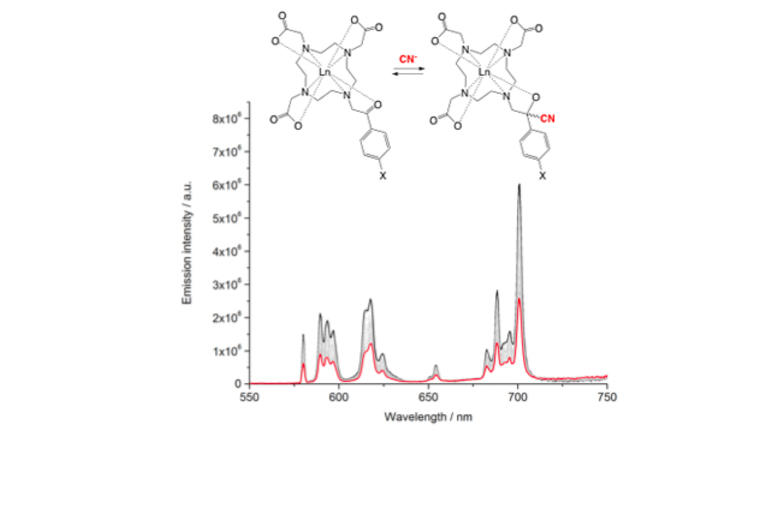
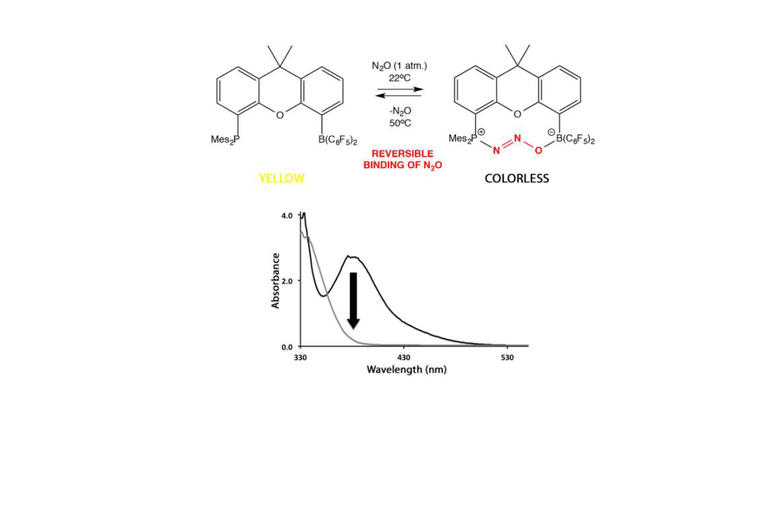
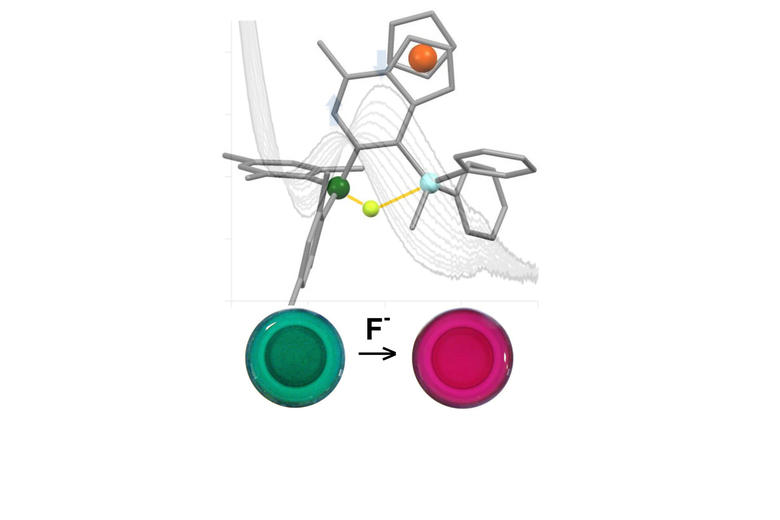
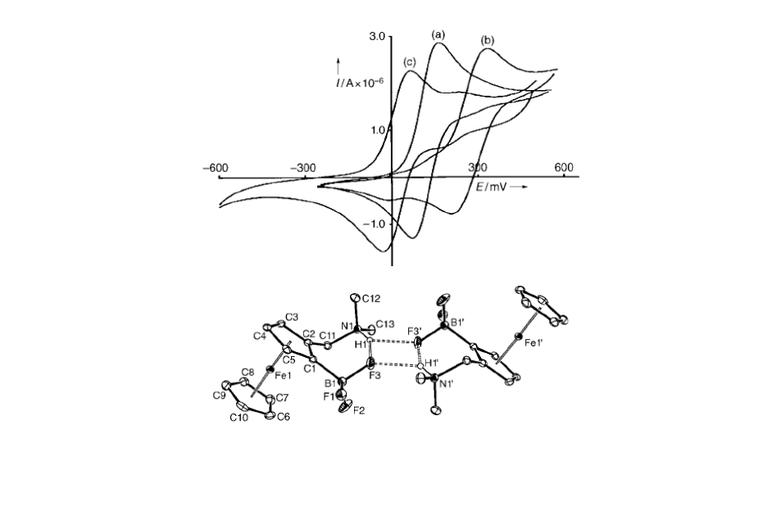
A wide variety of chemical strategies have been reported in the literature to selectively bind anions, neutral Lewis bases and whole acids, with a view to developing detection/sensor technologies. We have a longstanding interest in the use of group 13 Lewis acids for this purpose, with selectivity based either on the strength of the donor/acceptor bond formed with the analyte (e.g. for fluoride) or on the complementary geometry of the binding site and target anion. Recent work in this area has focussed on multifunctional Lewis acids and mixed Lewis acid/base systems for the selective detection of fluoride and cyanide (or HF/HCN) in the presence of competitive anions (e.g. halides). More recently, we have been using 'frustrated' Lewis acid/base pairs for the activation and detection of difficult analytes (including volatile atmospheric oxides such as nitrous oxide) which have low affinities for more conventional receptor systems. A key design principle in much of this work is the incorporation of ferrocenyl units for electrochemical or colorimetric reporting. More recently, in collaboration with the Faulkner group we have been developing systems which offer a fluorescence based assay.
From an applications perspective this work has generated a simple colorimetric swab technology for the detection of toxic environmental contaminants (including chemical warfare agents). Our work on fluoride binding/uptake has also led to a new CRUK-funded project on new methodologies for the delivery of 18F for PET imaging.
Selected publications: