Main Group Bond Activation
Main Group Bond Activation
Moulding the electronic structure of p-block compounds for small molecule activation
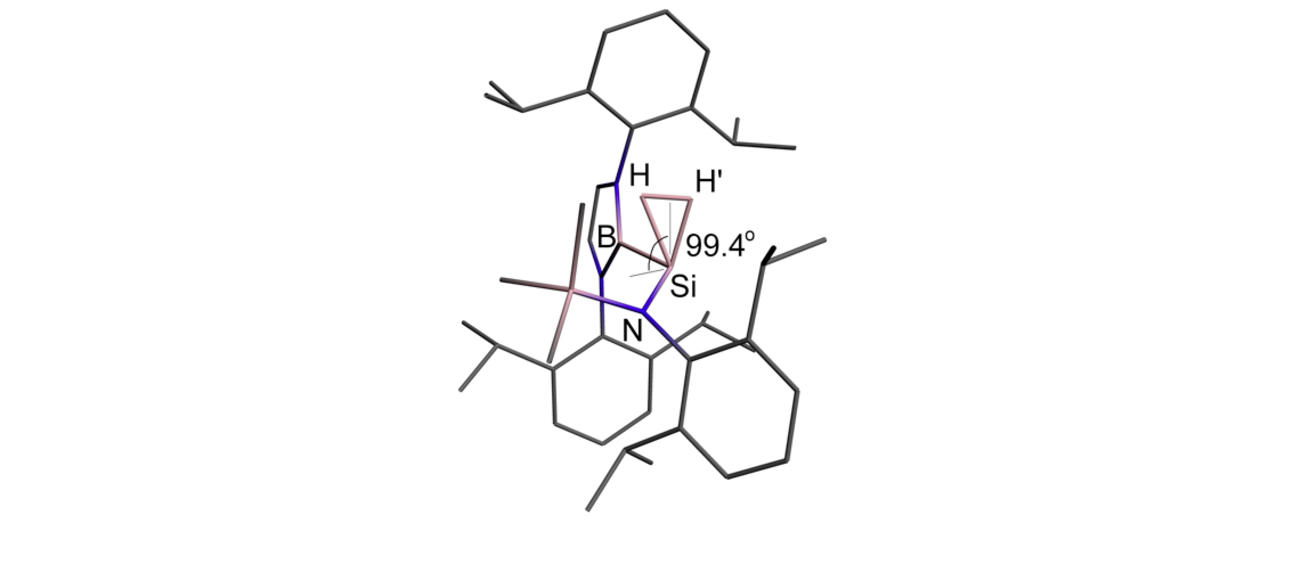
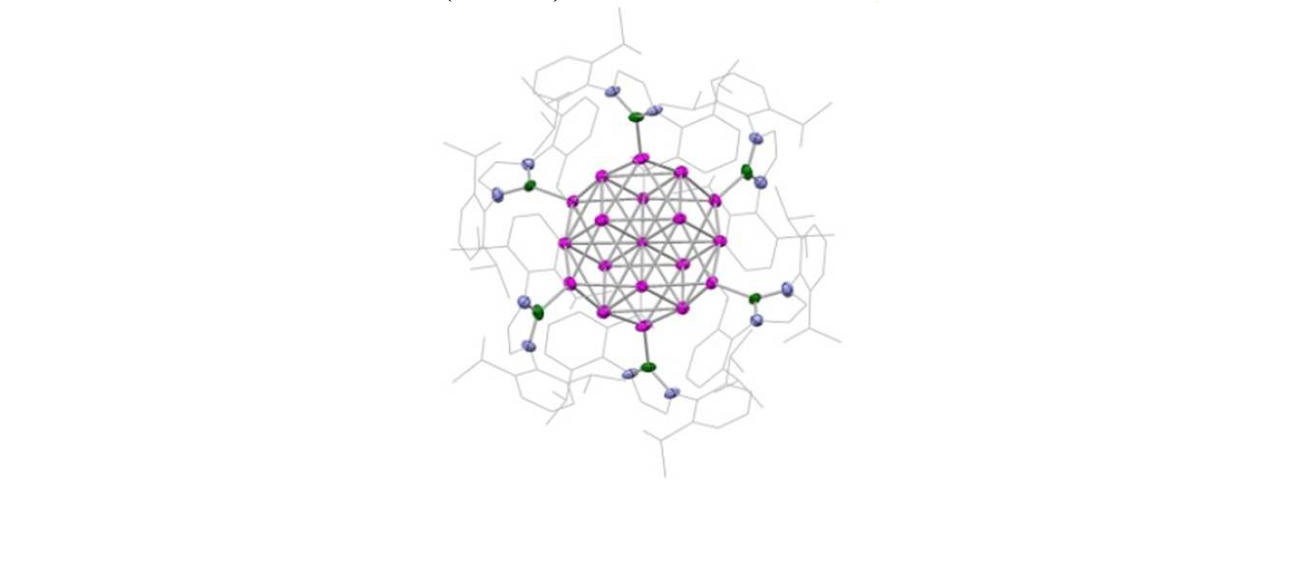
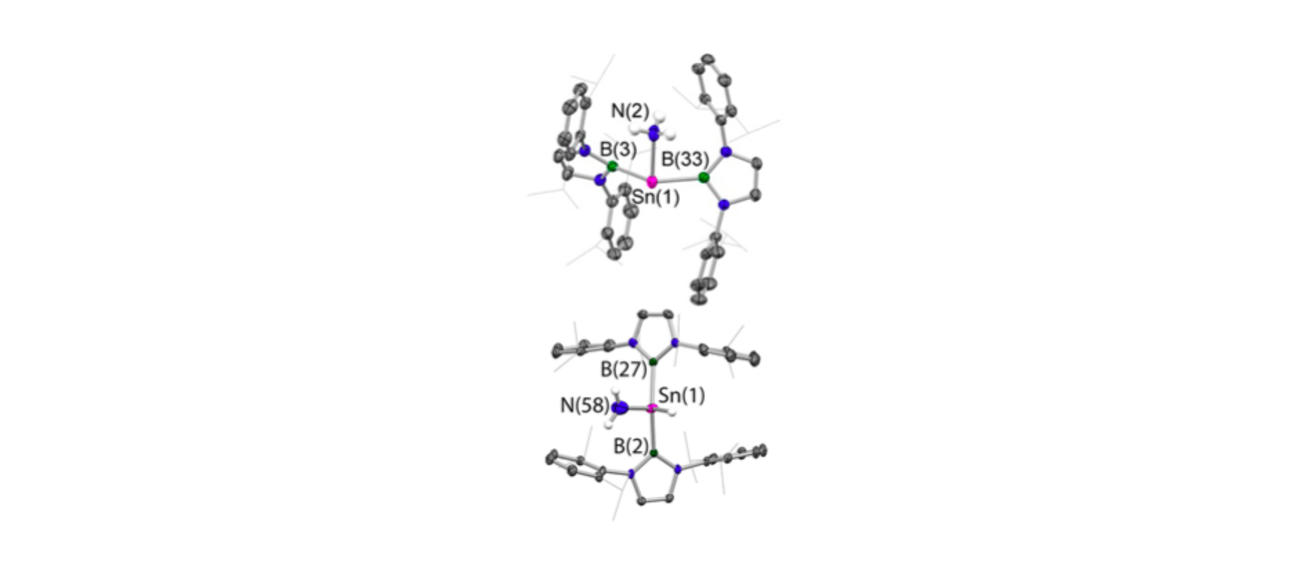
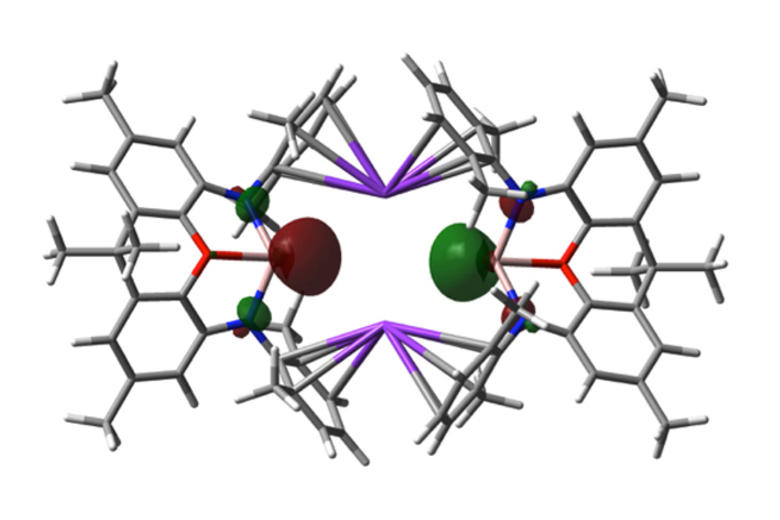
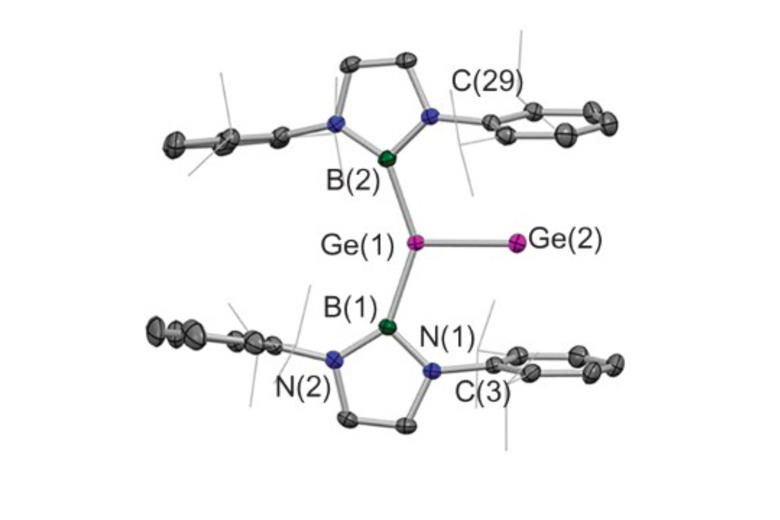
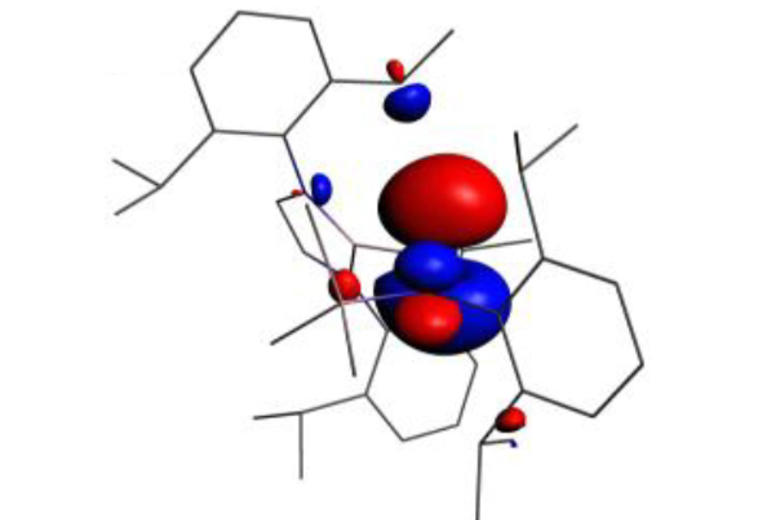
Much of this work has focussed on the design and synthesis of p-block compounds which possess ‘transition metal like’ attributes critical for the activation of small molecules, namely: (i) small HOMO-LUMO gaps; (ii) high energy HOMOs for the activation of kinetically inert bonds; (iii) unpaired electrons; and/or (iv) redox properties appropriate for reversible bond formation. This work has led to the development of novel systems for the activation of small molecules such as dihydrogen, ammonia, carbon monoxide/dioxide and hydrocarbons such as benzene (through cleavage of both C-H and C-C bonds).
Highlights include: (i) nucleophilic compounds of aluminium that open up a new type of chemistry to one of the more technologically important elements, via umpolung (reverse polarity) bond-forming substitution processes; (ii) the activation of H2 by unprecedented silylene and heavier vinylidene compounds; (iii) the first example of C-H oxidative addition of benzene by a Main Group compound; and (iv) the first example of activation of the C-C bond in benzene by any isolable metal compound; (v) mechanistic studies of the functionalization of ammonia by a Main Group compound via redox chemistry - showing experimental evidence for an alternative (coordination, proton-migration) mechanism for N-H oxidative addition; and (vi) reversible M-C bond formation at a Main Group metal centre.
Selected publications: